
Historically, immunotherapy has demonstrated limited effectiveness in a significant majority of patients with colorectal cancer. However, promising findings from an investigator-initiated trial conducted at NewYork-Presbyterian/Weill Cornell Medicine have shown that neoadjuvant immunotherapy is efficacious in treating resectable colorectal cancer.
While patients with mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) colorectal cancer are exquisitely responsive to commercially available immunotherapy drugs, this population accounts for only 5-15% percent of all patients with colorectal cancer. The other 85-95% of colorectal cancer patients are mismatch repair proficient/microsatellite stable (pMMR/MSS), and a proportion remains uncured despite surgery and chemotherapy. Thus, there is a critical need for more effective therapies for most patients with the more common type of “cold” colorectal cancer that does not respond to traditional immunotherapy drugs.
Previous data from a phase 1a/1b study showed that treatment with the multifunctional next-generation anti-CTLA-4 antibody botensilimab (BOT) plus the anti-PD-1 antibody balstilimab (Agenus Bio Inc.) had a 22% response rate in patients with heavily pretreated pMMR/MSS metastatic colorectal cancer.3 Additionally, neoadjuvant therapy has shown great promise in cancer and presents an ideal setting to evaluate new drugs. The NICHE-1 trial demonstrated the curative activity of neoadjuvant immunotherapy in metastatic dMMR/MSI-H colon cancer. These two things led to the genesis of the NEST-1 Clinical Trail.
NEST-1 Clinical Trial Shows Promising Results
Pashtoon Kasi, MD, MS, Director of Colorectal Cancer Research at NewYork-Presbyterian/Weill Cornell Medicine [and the Englander Institute for Precision Medicine's Director of Director of Colon Cancer Research and Director of Liquid Biopsy Research] is the principal investigator of the NEST-1 clinical trial, a single-arm trial evaluating neoadjuvant BOT/BAL in patients with resectable colorectal cancer. Given that neither of these drugs has been tested in the neoadjuvant or pre-surgery setting, the trial included both pMMR/MSS and dMMR/MSI-H colorectal cancer patients. A maximum of 25% of patients were allowed to be dMMR/MSI-H since the focus was the bigger unmet need for immunotherapy options for patients with pMMR/MSS “cold” colorectal cancer.
The study included 12 patients with colon and rectal cancer. Nine patients had pMMR/MSS colorectal cancer, and 3 had dMMR/MSI-H. The study included a diverse group of patients representative of the New York population. All patients received a single dose of BOT 75 mg and two doses of BAL 240 mg 2 weeks apart. One week after the second dose of BAL, patients were able to proceed to any standard surgery for colorectal cancer. Changes in minimal residual disease were assessed pre- and post-surgery using circulating tumor DNA (ctDNA).
One of the barriers in recruiting for this trial was that it challenged the existing treatment paradigm of surgery then chemotherapy. Further, while many patients want to get rid of cancer as soon as possible, this protocol necessitated that patients delay their surgery. “The current practice is you do your surgery first, you do chemotherapy later, and nothing before that,” says Dr. Kasi. “Some of these patients are not even seen by medical oncologists like me. So, this was an important barrier and challenge for us to address to be able to work in a multidisciplinary way so these patients were at least seen by us in medical oncology prior to surgery to learn about the study.
“Among our group of nine colorectal surgeons, they were eager [to refer patients to us] and every single patient that we talked to was open and comfortable delaying their surgery by a few weeks to sign up for the study,” he adds.
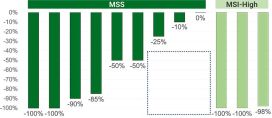 Results from the study were recently presented at the ASCO 2024 Gastrointestinal Cancers Symposium. The study met its primary endpoint. Sixty-seven percent of patients with pMMR/MSS colorectal cancer had a pathological response ≥50%. One hundred percent of patients with dMMR/MSI-H colorectal cancer had a major pathologic response ≥90%. Reductions in ctDNA correlated with pathological responses. One hundred percent of patients positive for ctDNA at screening cleared ctDNA, and 100% tested post-operatively have remained ctDNA negative. Additionally, none of the patients’ surgeries were delayed and none experienced immune complications that led to any treatment delays.
Results from the study were recently presented at the ASCO 2024 Gastrointestinal Cancers Symposium. The study met its primary endpoint. Sixty-seven percent of patients with pMMR/MSS colorectal cancer had a pathological response ≥50%. One hundred percent of patients with dMMR/MSI-H colorectal cancer had a major pathologic response ≥90%. Reductions in ctDNA correlated with pathological responses. One hundred percent of patients positive for ctDNA at screening cleared ctDNA, and 100% tested post-operatively have remained ctDNA negative. Additionally, none of the patients’ surgeries were delayed and none experienced immune complications that led to any treatment delays.
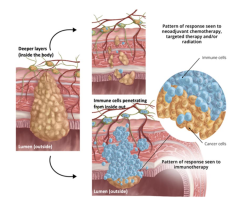 Results from this study also demonstrated a robust immunogenic pathologic response characterized by an inside-out (serosa-to-mucosa) pattern of regression where immune cells infiltrate from the inside, resulting in tumor cells being confined to the luminal surface. This was important spatial biology work highlighting many intriguing findings using RareCyte Inc. (Seattle, WA) Orien’s platform.
Results from this study also demonstrated a robust immunogenic pathologic response characterized by an inside-out (serosa-to-mucosa) pattern of regression where immune cells infiltrate from the inside, resulting in tumor cells being confined to the luminal surface. This was important spatial biology work highlighting many intriguing findings using RareCyte Inc. (Seattle, WA) Orien’s platform.
“What we hope to do in the long run is to cure more patients with this novel immunotherapy and move it up the ladder of the treatment paradigm,” says Dr. Kasi. “Not when options are limited, and all chemotherapy stops working, but before anything even happens, before even the surgery happens. That is the new neoadjuvant paradigm shift happening across solid tumors,” Dr. Pashtoon Kasi.
Last month, the NEST-1 clinical trial reopened. The expansion will include 12 more patients with dMMR/MSI-H colorectal cancer and 12 more patients with pMMR/MSS colorectal cancer. The goal of the expansion is to evaluate an extended 8-week course of immunotherapy to see if it would further deepen the responses and the necessity for surgery in patients with dMMR/MSI-H colorectal cancer.
# # #
The above artical originally appeared on the NewYork-Presbyterian's Advances website in April of 2024.
